NeuroMetrix maintains an active R&D program. Our strategy is to develop unique, proprietary medical devices that utilize non-invasive neurostimulation to diagnose and treat neurological conditions and pain. We seek to define new product categories and leapfrog existing technology. We develop products using our specialized in-house 10-person R&D team.
Diagnostics
-
DPNCheck® 2.0 launched in Q3 2022
-
DPNCheck® Cloud under development
-
Enhanced EHR integration
Therapeutics
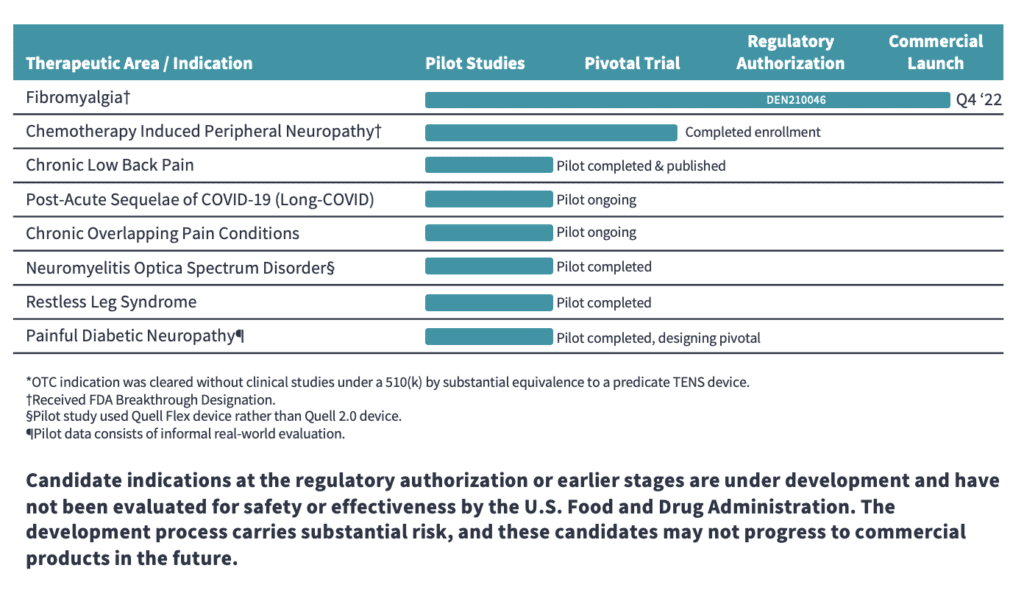
The Quell® technology currently has the following indications:
-
reduction of chronic lower extremity pain (over-the-counter)*
-
as an aid for reducing the symptoms of fibromyalgia in adults with high pain sensitivity (prescription)
We are seeking to expand the FDA authorized clinical indications to include a number of chronic pain and neurological conditions with high prevalence and unmet treatment needs.